Explain How a 1s Orbital Differs From a 2s Orbital
The 2s orbital can have more values of the ml quantum number than the 1s can. The 1s orbital is always filled before any other orbital.
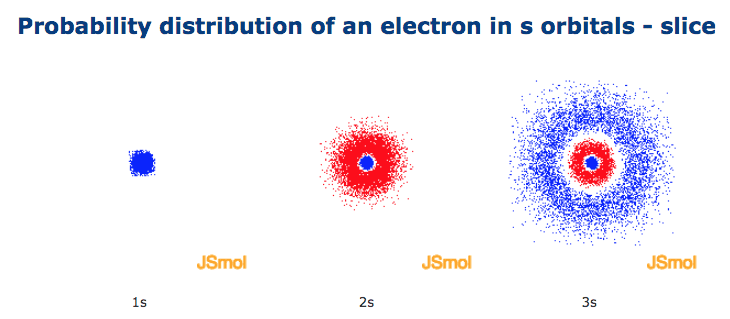
A Probability density plots of 1s and 2s atomic orbitals.
. Consider the difference between a 1s and 2s orbital. The 2s orbital extends farther from the nucleus than the 1s. Mas os elétrons são.
The 1s orbital has a higher energy than the 2s orbital. An orbital can contain a maximum of two electrons one spin-up and one spin-down. The 1s orbital has a higher energy than the 2s orbital.
1s and 2s are the sub-orbitals that are located in an atom. This means that the probability of finding the electron at a given distance is equally uniform in all directions. The second shell has two subshells.
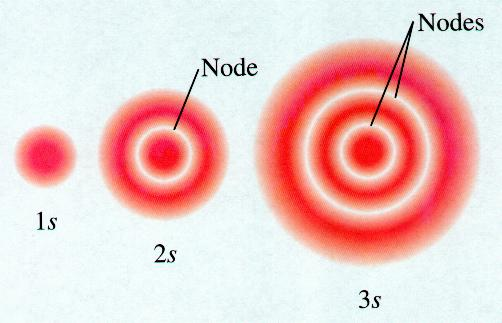
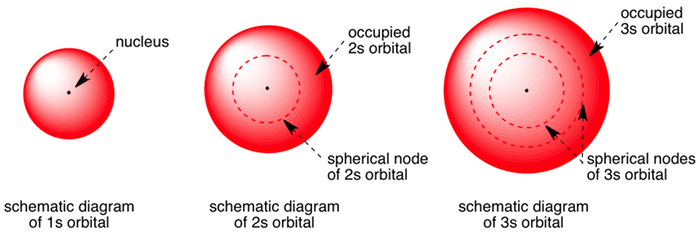
The 2s has more spherical radial nodes. An illustration of the shape of the 1s 2s and 3s orbitals. 2s orbital has higher energy than 1s.
1s- orbital is spherical. Therefore since n increased by 1 2s orbitals have one more. S orbitals are spherical in shape and increase in size as the energy level or shell increases.
This formation minimizes electron. The 2s orbital would be the same shape as the 1s orbital but would be larger in size and the 3p orbital would have the same shape as the 2p orbitals bout would be larger in size. Sp Hybridization can explain the linear structure in molecules.
Start your trial now. They are nearest to the nucleus and are found on the s sub-orbital. Hydrogen has one electron.
This is called the Pauli Exclusion Principal. The s sub shell can hold a maximum of two electrons as there is only one orbital. The density of the dots represents the probability density of finding the electron in that region.
Same goes for the 3s orbital 4s etc. Diferença-chave - 1s vs 2s Orbital. The difference between 1s and 2s is the difference in their level of energy.
The 1s has fewer nuclear nodes. First week only 499. This is designated as 1s 1 where the superscripted 1 refers to the one electron within the 1s orbital.
The 2s orbital is better at screening the outer orbitals than the 1s orbital. Um átomo é composto de partículas subatômicas principalmente prótons elétrons e nêutrons. The 2s orbital is better at screening the outer orbitals than the 1s orbital.
The size of the 2s orbital is larger than that of the 1s orbital. It is also possible for the orbital to have different kinds of. Protons e elétrons fazem o núcleo que está localizado no centro do átomo.
The number of angular nodes is given by l the angular momentum quantum number so the number of radial nodes is n - l - 1. The 2s has more spherical radial. The 2s orbital would be the same shape as the 1s orbital but would be smaller in size and the 3p orbitals would have a different shape than the 2p orbitals but would be larger in size.
This is designated as 1s 2. The first shell only has one subshell in it containing one orbital so it can contain a maximum of two elections. Describe Rutherfords model of the atom and compare it to the model of his student Niels Bohr.
1s orbital has the lowest energy because it is located closed to the nucleus. Weve got the study and writing resources you need for your assignments. Choose all that apply The 1s orbital has a different value for the l quantum number.
1s and 2s orbitals belong to different energy levels. Em outras palavras toda a matéria é feita de átomos. 1s and 2s orbitals occupied 2 electrons each and are spherical in nature.
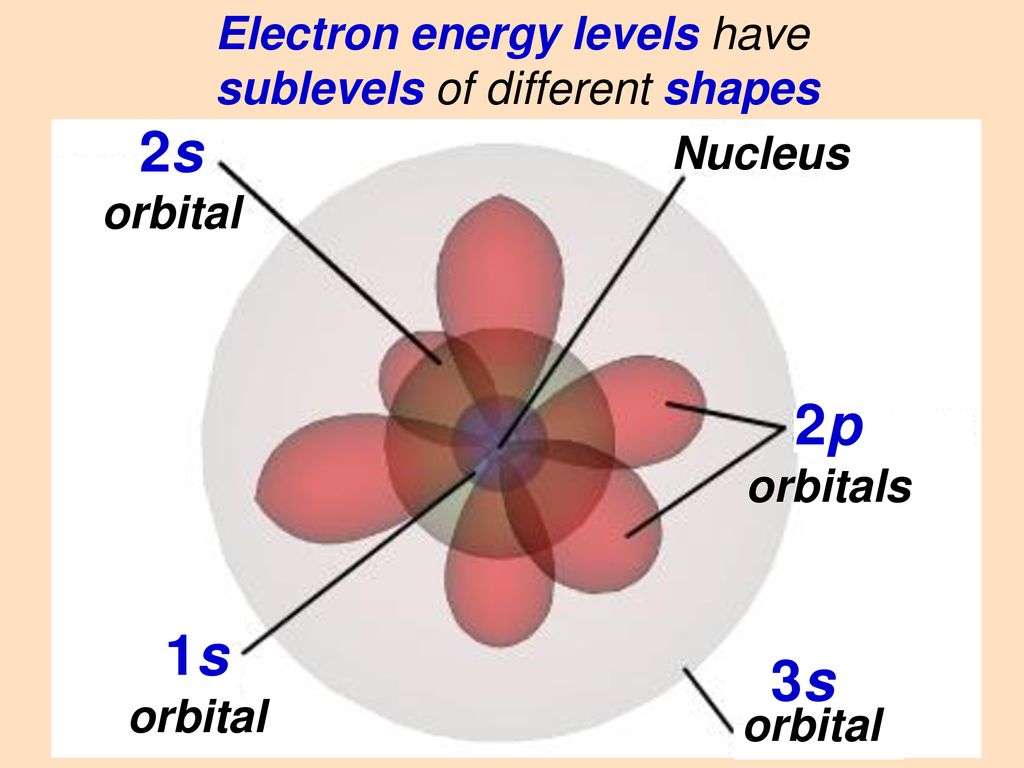
The 1s orbital is a sphere and the 2p orbital is made up of three dumbbells oriented in the x y and z direction. Helium has two electrons. My chemistry book explains that even though electrons in the mathrm2p orbital are closer to the nucleus on average electrons from the mathrm2s orbital spend a very short time very close to the nucleus penetration so it has a lower energy.
The 1s and 2s orbital are spherical in shape but the s orbitals are in general spherical symmetric. An orbital is an uncertain region where the possibility of the presence or locating of an electron is maximum. Also the 2 s and 3 p orbitals would have more nodes.
2p- orbitals are dumbbell shaped and oriented at right angles to each other. This is because the 2s orbital size resides farther away from the nucleus when compared to that of the 1s orbital. The 2s orbital extends farther from the nucleus than the 1s.
Therefore it has only one spot within the 1s orbital occupied. One has one orbital in it so a total of 2 electrons and the. The 1s has fewer nuclear nodes.
Solution for How is a 2s orbital different from a 1s orbital. A 2s orbital has one more radial node. 2s- orbital is spherical with a diameter larger than that of the 1s orbital.
Why does this tiny amount of time spent close to the nucleus make such a big difference. The front lobes face away from each other and form a straight line leaving a 180 angle between the two orbitals. The whole ideadesign doesnt change really only the possible areas at which the Electron might be in is greater.
2S orbital contains a nodal plane where 1s orbital does not have any node. Therefore it can completely fill the 1s orbital with its two electrons. 1s has low energy as compared to 2s.
The Shape of p. There is a 3-D space surrounding the nucleus that is actually representative of the orbital. In it the 2s orbital and one of the 2p orbitals hybridize to form two sp orbitals each consisting of 50 s and 50 p character.
Rutherford suggested the atom had a dense positively charged nucleus. O átomo é a menor unidade de matéria. The number of total nodes is n-1 where n is the principal quantum number n 1 2 3.
But for s orbitals l 0 so n - 1 n - l - 1 for s orbitals. The 1 shows the orbital is within the energy level closest to the nucleus while the s describes the shape of the orbital spherical for S.
Solved Hello I Do Not Understand Why There Would Be A Node Chegg Com
2s Orbital Contains 1s Orbital So If An Electron Is In 1s Is It Also In 2s How Do You Determine Quora
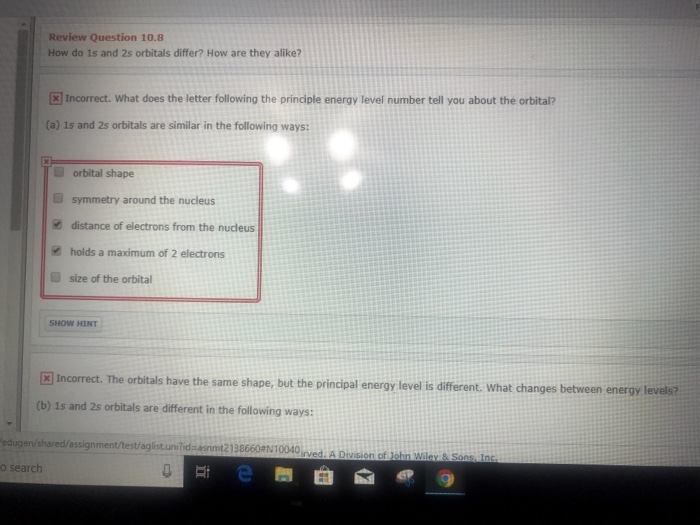
Solved Review Question 10 8 How Do 1s And 2s Orbitals Chegg Com

What Is The Difference Between 1s And 2s Orbital Quora
What Is The Difference In The Shapes Of 1s And 2s Quora

Chemistry The Central Science Chapter 6 Section 6

Solved What Is The Difference Between A 3s Orbital And A 2s Chegg Com

3 The Overlay Of 1s And 2s Orbital Which Were In The Different Energy Download Scientific Diagram

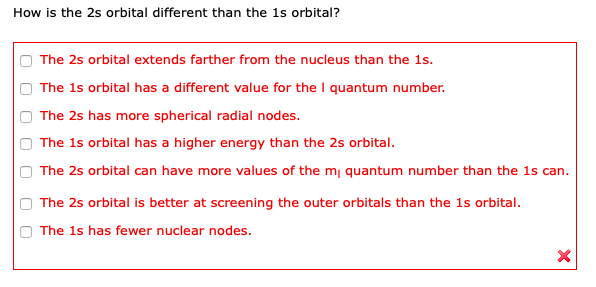
Solved How Is The 2s Orbital Different Than The 1s Orbital Chegg Com

Write 2 Similarities And 2 Differences Between 1s And 2s Orbitals Brainly In

Solved The Major Difference Between A 15 9 Orbital And A Chegg Com

Difference Between 1s And 2s Orbital Compare The Difference Between Similar Terms

A 1 Molecular Orbital Theory Chemistry Libretexts
Electron Configurations Ppt Download
Can The 2 Electrons Of 2s Orbital Touch The Sphere Of 1s Orbital Quora


Comments
Post a Comment